Bioeconomy Reading time 2 min
The valorisation of animal biomass: when by-products are worth their weight in gold!
Published on 27 January 2020
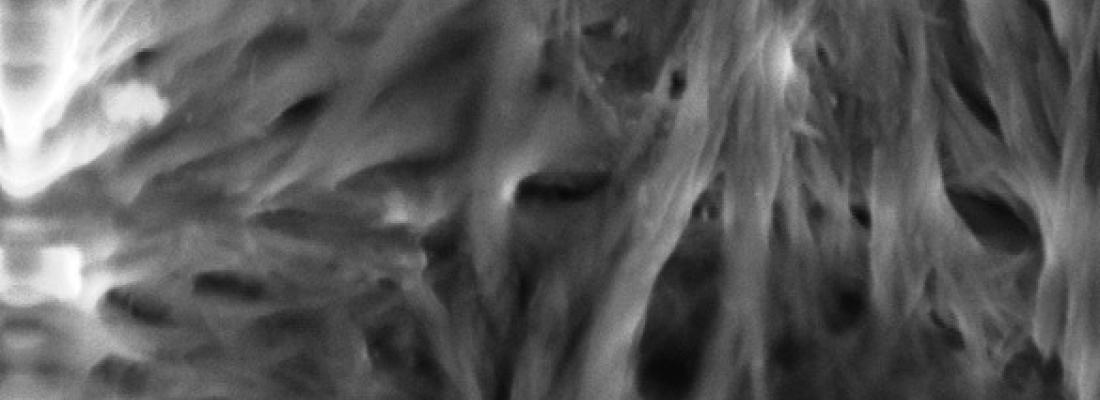
In animals, structural fibrous proteins – collagen, elastin and keratin – are involved in the stability and mechanical resistance of tissues, protection against pathogens and numerous physiological pathways. Because they are present in all tissues, collagen, elastin and keratin can be extracted from the by-products that are generated in large quantities by abattoirs in the meat industry.
The very first publication by a French research institution on the valorisation of animal biomass concerning the recovery of structural fibrous proteins appeared in 2017. France was one of the last of the most industrialised countries on the planet not to valorise this biomass which has considerable biological and technical potential, despite the importance of this resource as well as the large number of its potential applications.
The first results concerned the extraction of fibrillar collagen from bovine bones, taking account of several parameters such as the age and type of bovine bone or the drying technique employed, in order to study the assembly of the collagen thus extracted. These factors are closely linked to the ex vivo functionality of collagen and may therefore determine its potential applications. Further studies enabled the analysis of its denaturation equilibrium and determination of the parameters linked to its structure.
The valorisation of animal biomass offers an opportunity for change and an economic revolution, as well as sustainability in several sectors. It may be possible to replace numerous materials of synthetic origin, or produce biologically active components (antioxidant, anti-hypertensive, anti-diabetic, anti-inflammatory, etc.) thanks to the properties of collagen, elastin and keratin.
References
Ferraro, V., Gaillard-Martinie, B., Sayd, T., Chambon, C., Anton, M., Santé-Lhoutellier, V. (2017). Collagen type I from bovine bone. Effect of animal age, bone anatomy and drying methodology on extraction yield, self-assembly, thermal behaviour and electrokinetic potential. International Journal of Biological Macromolecules, 97, 55-66.
Ferraro, V., Anton, M., Santé-Lhoutellier, V. (2016). The “sisters” α-helices of collagen, elastin and keratin recovered from animal by-products: functionality, bioactivity and trends of application. Trends in Food Science & Technology, 51, 65-75.