Food, Global Health Reading time 8 min
Clinic’n’Cell - Ex vivo clinical trials - a start-up for animal-free testing of health products
Published on 06 September 2021
Various assertions are made about consumer health products: for example, they may be said to protect against osteoarthritis, control cholesterol, or boost antioxidants. How do we verify the veracity of these claims for cosmetics or food supplements? Currently, a lengthy R&D process is used: it starts off with in vitro studies in cells, continues on to animal testing, and finishes up with clinical studies in humans.

The question arises: could there be more ethical and effective alternatives? In 2015, Fabien Wauquier and Yohann Wittrant began seeking the answer to this question. The two are cell biologists who built their careers studying the links between food and health at the Joint Research Unit for Human Nutrition (INRAE, University of Clermont-Ferrand). Their project was formalised after they met Line Boutin, who was working at that time at the Auvergne Centre for Human Nutrition Research (CRNH-A). Boutin, Wauquier, and Wittrant now offer companies a new way to test health products. Their clinical approach is ex vivo, Latin for “outside the living”, and is intended to be faster and more accessible than classical clinical research in humans. It also takes a more physiological tack than in vitro strategies and is more ethical than animal experimentation. Below, the three researchers tell us more.
What sparked the birth of Clinic'n'Cell?
Yohann Wittrant: Clinic’n’Cell arose from a simple idea: when we ingest foods, supplements, or medications, digestion follows. The resulting compounds enter the bloodstream and then affect all body cells. We collect blood samples containing these compounds from study volunteers and apply the blood to cultured human cells. Within a few weeks, we can determine whether the test product exerts its intended effect. We can thus forgo animal testing but still obtain data that reveal the molecular mechanisms at play.
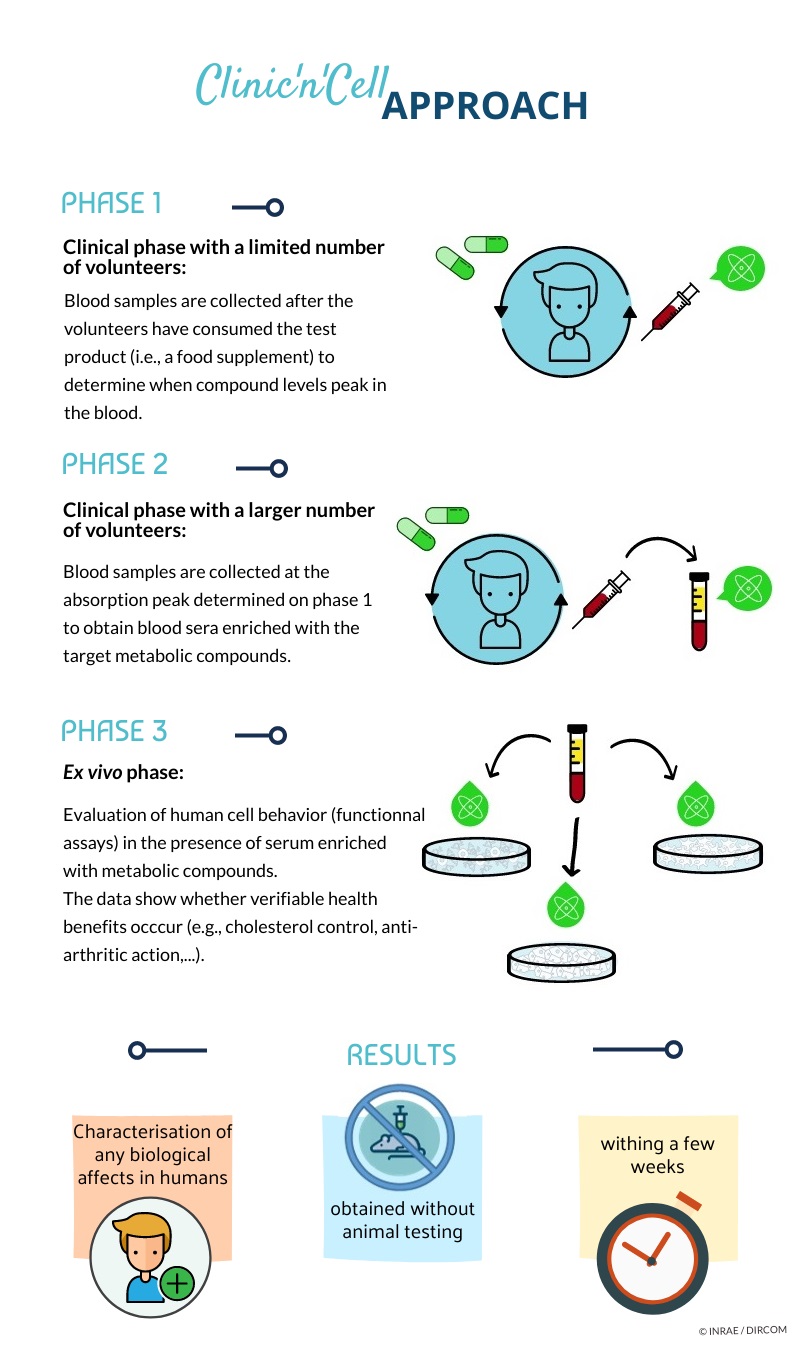
Clinic’n’Cell arose from the industry’s desire for a more ethical approach in nutrition and health research. It also meets an academic need: scientists require models that better represent human physiology, which is a current weakness of traditional approaches such as in vitro cell culturing or rodent-based experimentation.
Fabien and I initially came up with the idea in 2015. Then, in 2016, we presented our project proposal to La Maison Innovergne, an alliance of public stakeholders that promotes innovation. The group gave us funding for our market study. Our project had potential, but the market was not ready for us. We also needed to refine our methodology. Fabien and I had the requisite expertise in cell biology, but we needed a collaborator with a clinical background, especially because of the regulatory concerns related to research involving human volunteers. This type of work takes substantial planning! That is when Line joined us. She has 10 years of experience as the assistant director of the Auvergne Centre for Human Nutrition Research. We were able to jointly fund the start-up’s development in 2018 and 2020 thanks to INRAE Transfert and collaborations with companies in the food, nutraceutical, food supplement, and nutricosmetics industries. Our work during this period allowed us to enter the market in March 2020, offering a proven methodology that could immediately meet our clients’ needs. We simultaneously continued to publish our research results.
Are you still affiliated with INRAE?
Fabien Wauquier: We are still involved in academic research. We developed our method in a publicly funded laboratory, and our work gave rise to a know-how license, which we are currently utilising. Our start-up is hosted by a laboratory in the Joint Research Unit for Human Nutrition (INRAE, University of Clermont-Ferrand), where we carry out our work with cells. I focus 100% on the start-up, but Yohann has largely maintained his role as a researcher. He dedicates around 20% of his time to our scientific consulting services, presenting the start-up’s approach. We are still scientists, and we are striving to make our work available to as many people as possible. If we had continued to work exclusively as public-sector researchers, we would not have been able to consistently offer our private services.
What are your plans for the future?
We want to make Clinic’n’Cell an R&D industry standard
Line Boutin: The Clinic’n’Cell approach is protected as confidential know-how by INRAE and the University of Clermont-Ferrand. We thus have no direct competitors. For the moment, our main objective is to promote our approach’s use among the food sector’s major players, such that it becomes an R&D industry standard, on par with the others currently used to evaluate the physiological effects of test products. This work involves spreading awareness of our testing system. The pandemic has not made this task easy, especially because scientific conferences were cancelled. However, the industry is starting to talk about us, and we are thinking about restructuring the company (e.g., facilities, equipment, personnel) if demand for our services keeps growing. We are receiving more and more requests from cosmetics and nutricosmetics manufacturers, who want to eschew animal testing for ethical reasons and thus meet consumer demand for more natural products. Large companies that have been doing R&D for a long time want to limit the risks associated with putting new products on the market. In such situations, our approach could serve to bridge the gap between obtaining promising results in animal models and launching long-term clinical testing in humans. We are also developing internationally and have our first contract in the Netherlands. Looking forward, we want to see if the pharmaceutical industry might be interested in our approach. For example, Clinic’n’Cell could help determine whether approved medications also have further alternative beneficial effects.

Clinic’n’Cell has received the Innovation Award in the 2022 NutraIngredients Awards for Europe. For more information (in French).
The START-UP in figures
- 2 salaried employees
- 1 know-how license protected by INRAE and the University of Clermont Auvergne (DIRV#18-0058).
- 7 clinical validation studies & 4 related publications (2019–2021)
- 4 contracts established during the start-up’s first year (2020)
- Recipient of a 2021 Bourse French Tech from BpiFrance
- Services of ensured scientific quality
- 1 start-up meeting the needs of the dietary supplements industry, whose worldwide turnover is expected to reach €450 billion in 2020 (+8% per year)

Results of an innovative ex-vivo clinical trial demonstrating the beneficial properties of an artichoke leaf abstract. Read on (in French only).
