Food, Global Health Reading time 3 min
A miniature human liver to transform toxicology testing of food contaminants
PRESS RELEASE - An INRAE research team has developed a microscopic model of the human liver to study the effects of different food contaminants. The spherical model, measuring 0.3 mm in diameter, includes the four cell types found in the human liver, unlike most current laboratory tests, which rely on a single type of cell. The researchers have designed an innovative process to assess the toxic effects of a contaminant across the entire 3D model and pinpoint the location and types of cells that are affected. Published in NAM Journal, the findings are expected to improve health risk assessments for food contaminants by enabling testing at more realistic doses and reducing the need for animal testing. The work also opens up wider prospects in medicine, notably for drug development.
Published on 23 January 2026
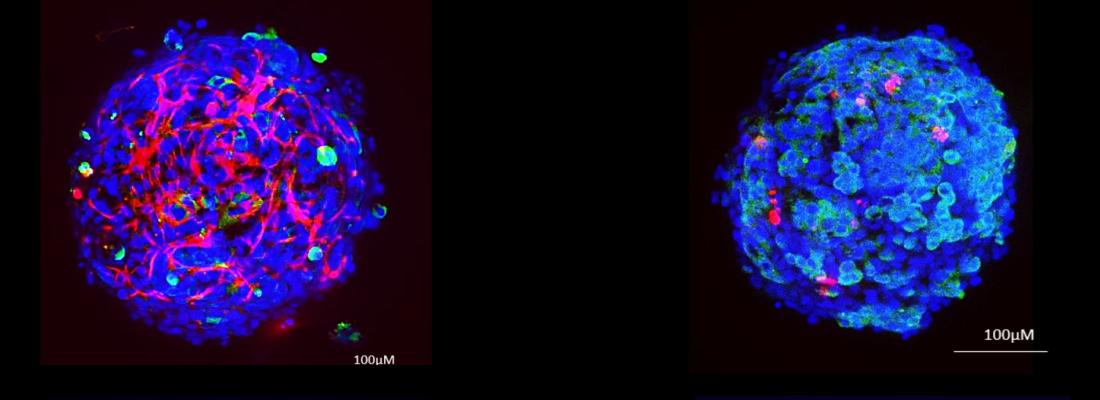
Assessing the toxicity of food contaminants—including carcinogenic potential—is a major challenge in evaluating the risks associated with exposure. In recent years, as part of efforts to reduce animal testing, two-dimensional (2D) analytical methods using human hepatic cell lines (which make up most of the liver) have advanced predictive toxicology for contaminants. However, these approaches have limitations, because they do not sufficiently capture the organ’s complexity. To improve toxicology studies of chemicals, the researchers developed a miniature, three-dimensional (3D) liver model that includes the different cell types that make up a human liver.
A human-relevant mini-liver for toxicology studies
In laboratories, human cell lines are typically grown in two dimensions as a flat layer at the bottom of culture dishes. The team has developed a new three-dimensional, spherical cell culture model that enables several cell types to be grown together to mimic the structure of a human liver. This tiny 0.3-mm sphere contains around 2,000 cells and includes all four types of cells found in the human liver: hepatic cells (hepatocytes), cholangiocytes, stellate cells and immune cells*. The mini liver demonstrates physiological and metabolic capabilities that mimic those of a human liver and are more representative than the models traditionally used (i.e. cell culture and animal models).
An innovative method for 3D toxicology analysis
Using this mini-liver, the research team developed an innovative confocal microscopy process that is high-throughput and high-resolution. For the first time, toxicology analyses can be conducted at single-cell scale within the mini-liver model. The method enables the detailed and simultaneous analysis of several contaminant-induced effects on cells, including DNA damage, the cell proliferation that is characteristic of cancer cells, inflammation, and the accumulation of fats often seen with liver disease or obesity. Scientists revealed certain effects not detected by conventional 2D cell-based analysis, such as the spatial distribution of effects and the types of cells affected. This method also makes it possible to test much lower doses—more representative of real-life exposure—than those typically used in 2D cell analysis. The process has been optimised to run as many as 72 toxicology tests simultaneously using multi-well cell culture plates.
“This mini-liver model mimics the human organ. Our approach maps the effects of contaminants and reveals certain impacts that conventional methods do not detect. In the medium term, using this type of human cell model should improve our ability to predict the toxicity of certain chemicals in humans and limit the need for animal testing.” Marc Audebert, INRAE Research Director
Reference
Recoules C., Audebert M. (2026). Set up of a human 3D liver multicellular model for chemical high-throughput toxic hazard assessment. NAM Journal, 2, 100075 https://doi.org/10.1016/j.namjnl.2025.100075
Study carried out as part of the European Partnership for the assessment of risks from chemicals (PARC)
