Food, Global Health Reading time 3 min
Titanium dioxide: evidence of its toxicity
Published on 06 May 2021 (date.last_update 22 May 2023)
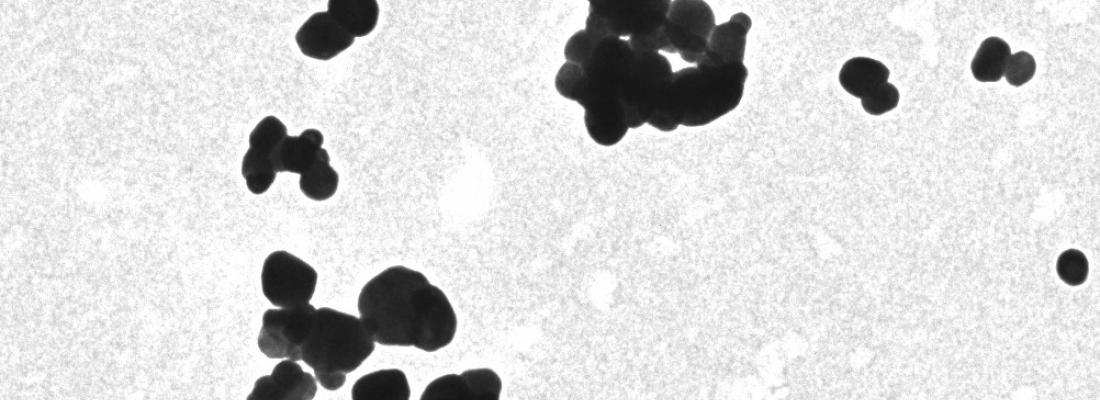
What is titanium dioxide?
It’s a food additive widely used throughout the world for its opacifying and colouring properties (white pigment). Better known in Europe as E171, the sale of food products containing this additive has been suspended in France since January 1st, 2020, for precautionary reasons[1] and its ban has just been extended to Europe in January 2022.
Studies by INRAE scientists in 2017 and 2020, first on the immunotoxic potential of E171 and its role in promoting precancerous lesions in the intestine then on titanium dioxide (TiO2) nanoparticles crossing the placental barrier, provided the scientific evidence necessary for these measures. In 2021, EFSA updated its previous evaluation published in 2016 on E171, highlighting data gaps in its previous assessment which had asserted the absence of a health risk. EFSA now considers that E171 is no longer safe when used as a food additive, an opinion which has led to a change in regulations at the European level.
These nanoparticles pass through the oral mucosa to reach the bloodstream, thus well before their absorption in the intestine, but also they can affect cell regeneration within these same mucosa. This work highlights the importance of taking into account direct exposure of the oral cavity to the food additive E171 when assessing risks to humans, both when used in food products and in cosmetics (particularly toothpaste) and pharmaceuticals.
Looking back on our key research results:

E 171 crosses the placental barrier in humans
Working in collaboration with the Laboratoire national de métrologie et d’éssais (LNE), the Groupe de Physique des Matériaux (CNRS/INSA Rouen/Université de Rouen-Normandie), Toulouse University Hospital, Picardie-Jules Verne University and the National Veterinary School in Toulouse, INRAE scientists have pursued their work in humans and have supplied proof that TiO2 nanoparticles present in the additive E171 can cross the placenta and reach the foetal environment. Their results, published on 7 October 2021 in Particle and Fibre Toxicology, alert on the importance of evaluating the risk of exposure of pregnant women to these nanoparticles.
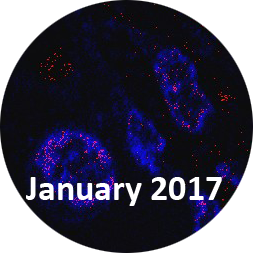
E171 crosses the intestinal barrier in animals
INRAE researchers and their partners have studied the effects of oral exposure to titanium dioxide, an additive (E171) commonly used in foodstuffs, especially confectionary. They have shown for the first time that nanoparticles of E171 cross the intestinal barrier in animals and reach other parts of the body. Immune system disorders linked to the absorption of the nanoscale fraction of E171 particles were observed. The researchers also showed that chronic oral exposure to the additive spontaneously induced preneoplastic lesions in the colon, a non-malignant stage of carcinogenesis, in 40% of exposed animals. Moreover, E171 was found to accelerate the development of lesions previously induced for experimental purposes. While the findings show that the additive plays a role in initiating and promoting the early stages of colorectal carcinogenesis, they cannot be extrapolated to humans or more advanced stages of the disease. The findings were published in the 20 January 2017 issue of Scientific Reports.
2017 press release
In 2018, researchers showed that titanium dioxide did not weaken the intestinal mucusa in animals. (2018 press release in French).
In 2020, the main absorption pathways of TiO2 in the intestine were identified by the same teams (DOI: 10.1186/s12989-020-00357-z).
1 Application of the French Law of 30 October 2018 on the balance of trade relations in the agricultural and food sector and healthy, sustainable, and accessible food for all (EGalim Law). This measure applies to food products sold in France, for a period of one year, potentially renewable. This precautionary principle does not apply to non-food products. https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000038410047?r=v1pKGxVbGN (in French)
