Food, Global Health Reading time 2 min
Diabetes and obesity: the disappearance of a glucose detector in the abdomen
Published on 26 October 2020

The detection of blood glucose is crucial. The first sensor is found in the hypothalamus in the brain, and mainly detects hypoglycaemia, while the second is in the abdomen at the level of the portal vein which drains blood from the digestive tract. Its principal role is to detect hyperglycaemia. However, the characteristics and variations of this sensor, particularly in obese or diabetic patients, were hitherto unknown.
To enable direct study of this sensor, the scientists synthesised a radio-labelled probe binding to it. They monitored the level of probe binding to the sensor using an innovative molecular imaging methodology (PET-CT – see insert). This was able to localise, quantify and measure the activity of the glucose detection sensor in the portal veins of miniature pigs before and after weight gain, up to the stage of pre-diabetes. In animals that were not overweight, the researchers were able to determine that the sensor mechanism extended over a short distance at entrance to the liver but not throughout the portal vein. In obese animals, the sensor had disappeared, thus preventing the detection of glucose in the abdomen. These findings were confirmed by recording the nervous signal arising from the same sensor and finding that the signal sent to the brain was indeed suppressed.
Disappearance of the portal sensor during weight gain removes crucial information for the brain which can no longer correctly evaluate blood glucose levels. This disappearance is probably implicated in the events that transform obesity into type II diabetes. However, its restoration appears to be possible insofar as the imaging methodology developed here enables the rapid and non-invasive monitoring of this sensor, which is a significant advance in personalised medicine. These findings offer new prospects for the prevention and treatment of obesity and diabetes.
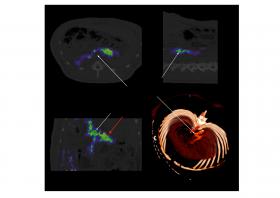
To detect glucose, the portal vein sensor uses receptors that detect a hormone (glucagon-like peptide 1). These receptors are also sensitive to other molecules, some of which are notably used to treat diabetes. It was by using a labelled probe (i.e. detectable using imaging technologies) binding to these receptors that the scientists were able to localise and measure the activity of the glucose sensor. During the same examination, hybrid PET-CT imaging was able to monitor the binding of this labelled probe proportionally to the density of receptors (PET image) in the patient’s body (CT image).
Figure: PET-CT image of the activity of portal vein sensors (in colour) in an animal model. Lower right: three-dimensional representation of the portal vein in the same animal. © INRAE / Charles-Henri Malbert |
|
References Charles-Henri Malbert, Alain Chauvin, Michael Horowitz, Karen L Jones, Glucose-sensing mediated by portal GLP-1 receptor is markedly impaired in insulin-resistant obese animals. Diabetes, Diabetes 2020 Oct, doi.org/10.2337/db20-0361 Jones KL, Huynh LQ, Hatzinikolas S, Rigda RS, Phillips LK, Pham HT, Marathe CS, Wu T, Malbert CH, Stevens JE, Lange K, Rayner CK, Horowitz M. Exenatide once weekly slows gastric emptying of solids and liquids in healthy, overweight people at steady-state concentration. Diabetes Obes Metab. 2020, 22: 788-797 doi: doi.org/10.1111/dom.13956. |
