Climate change and risks Reading time 3 min
Can ground voles transmit zoonoses?
Published on 24 February 2023

Outbreaks are common for several vole species, including the water vole (Arvicola terrestris) in France. Such events are part of a multiyear cycle, where a population outbreak is followed by a rapid bust. Voles are rodents, a group that frequently hosts zoonotic agents. Additionally, these species commonly colonise habitats where domestic ruminants occur in close contact with humans. Taken together, these three traits strongly increase the likelihood that A. terrestris could represent a major source of pathogens for animal and human populations. Led by the Research Network for Animal Health and Welfare in Auvergne Rhône Alpes (SAARA), pré-PATHOZOON used several molecular techniques, including next-generation sequencing, to characterise samples taken from A. terrestris populations in Auvergne. This work uncovered the presence of zoonotic bacteria and viruses alike.
The study sites were three livestock pastures that were also home to A. terrestris populations. Ecosystems were similar across the sites, which were located near the Auvergne Volcanoes Natural Park and contained neither hedges, nor forests, nor wetlands. Researchers captured 46 water voles. They collected samples of various tissues, including urine and blood, under strictly aseptic conditions. The samples were stored at -20°C until DNA and RNA extraction could occur.
Next, PCRs were performed that specifically looked for pathogenic Leptospira species, Coxiella burnetii, various coronaviruses, and SARS-CoV-2. The samples were also subject to random whole transcriptome sequencing (RNA-seq). While PCR analyses are quite sensitive, they are also highly pathogen specific. In contrast, next-generation sequencing methods can reveal the presence of unexpected pathogens, although their limited sensitivity means they will not pick up on any occurring at low levels. The study’s use of these two molecular approaches was thus complementary and constituted a key strength. Indeed, this work was unprecedented for its thoroughness.
High prevalence of Leptospira. A total of 36 water voles had at least one tissue test PCR-positive for a pathogenic Leptospira species. While leptospirosis is often benign in humans, infection can cause kidney failure or even death in 5–20% of cases.
Outbreaks are common for several vole species, including the water vole (Arvicola terrestris) in France. Such events are part of a multiyear cycle, where a population outbreak is followed by a rapid bust. Voles are rodents, a group that frequently hosts zoonotic agents. Additionally, these species commonly colonise habitats where domestic ruminants occur in close contact with humans. Taken together, these three traits strongly increase the likelihood that A. terrestris could represent a major source of pathogens for animal and human populations. Led by the Research Network for Animal Health and Welfare in Auvergne Rhône Alpes (SAARA), pré-PATHOZOON used several molecular techniques, including next-generation sequencing, to characterise samples taken from A. terrestris populations in Auvergne. This work uncovered the presence of zoonotic bacteria and viruses alike.
The study sites were three livestock pastures that were also home to A. terrestris populations. Ecosystems were similar across the sites, which were located near the Auvergne Volcanoes Natural Park and contained neither hedges, nor forests, nor wetlands. Researchers captured 46 water voles. They collected samples of various tissues, including urine and blood, under strictly aseptic conditions. The samples were stored at -20°C until DNA and RNA extraction could occur.
Next, PCRs were performed that specifically looked for pathogenic Leptospira species, Coxiella burnetii, various coronaviruses, and SARS-CoV-2. The samples were also subject to random whole transcriptome sequencing (RNA-seq). While PCR analyses are quite sensitive, they are also highly pathogen specific. In contrast, next-generation sequencing methods can reveal the presence of unexpected pathogens, although their limited sensitivity means they will not pick up on any occurring at low levels. The study’s use of these two molecular approaches was thus complementary and constituted a key strength. Indeed, this work was unprecedented for its thoroughness.
High prevalence of Leptospira. A total of 36 water voles had at least one tissue test PCR-positive for a pathogenic Leptospira species. While leptospirosis is often benign in humans, infection can cause kidney failure or even death in 5–20% of cases.
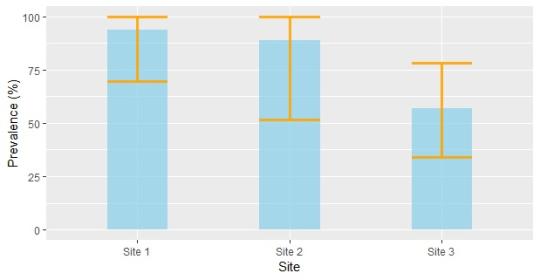
Leptospira prevalence in voles ranged from 57–94% across pastures. These figures far exceed those seen in other rodent species. For example, the brown rat (Rattus norvegicus) is considered to be the main reservoir host for Leptospira, even though its prevalence levels hover around 25%. The high Leptospira prevalence in water voles may stem from particular epidemiological circumstances. It could also be attributable to the swift sampling methodology, which reduced the likelihood of post-mortem contamination and thus made it easier to detect Leptospira DNA via PCR.
Absence of Coxiella burnetii and coronaviruses. Even armed with their powerful PCR technique, the researchers found no evidence of C. burnetii, which causes Q fever in ruminants and humans, or of any of the coronaviruses. Although C. burnetii has been observed in bank vole (Myodes glareolus) in France, it has yet to be seen in water voles. Coronaviruses are commonly found in a number of vertebrates and rodents. That said, none of the samples came up positive for any of the coronaviruses carried by voles.
Next-generation sequencing detected a smattering of zoonotic viruses belonging to seven different families. Among them was the weakly zoonotic Tula orthohantavirus, which occurred in three individuals from each of the pastures. This result suggests that the virus is circulating at low levels across all three sites. A virus in the genus Hepacivirus was found in four voles from the same pasture. This genus contains the hepatitis C virus that circulates in humans. The above discovery adds to the observations of Hepacivirus that have cropped up in recent years. These viruses have been detected in various animal species, including in rodents other than voles as well as in dogs, horses, and cattle. In-depth sequence analysis will reveal whether the virus in this study displays genomic similarities to viruses seen in other species, and particularly to those in murines.
To summarise, the two complementary sequencing approaches revealed that water voles can be infected with zoonotic bacteria in the genus Leptospira and with the weakly zoonotic Tula orthohantavirus. While the diversity of zoonotic agents was limited in the three water vole populations, Leptospira prevalence was remarkably high, a discovery highlighting that A. terrestris could play a major role in maintaining these bacteria in pasture ecosystems. Future research should look at other study sites.
In addition, to clarify how A. terrestris contributes to Leptospira epidemiology, it is essential to characterise spatiotemporal variation in infection patterns in populations of water voles and other hosts within the same habitat. Indeed, many zoonotic pathogens have multiple hosts and can infect several animal species, including wild animals, domestic animals, and humans. Such knowledge will inform measures for reducing zoonotic risks as different hosts may make different contributions to pathogen maintenance.
Research units involved in the pré-PATHOZOON project:
- VetAgro Sup: Research Unit for Wild Rodents, Health Risks, and Population Management (RS2GP; Florence Ayral, Elena Harran, and Virginie Lattard)
- INRAE: Joint Research Unit for Epidemiology of Animal and Zoonotic Diseases (EPIA; Xavier Bailly, Elsa Jourdain, and Julien Thézé), Research Unit for Wild Rodents, Health Risks, and Population Management (RS2GP)
- ANSES: Research Unit for Viral Genetics and Biosafety (Yannick Blanchard) and Research Unit for Lyssavirus and Wildlife Virology (Elodie Monchatre-Leroy)
With additional help from:
- Adrien Pinot, Marine Le Gudayer, Daouda Sionfoungo Soro, and Karine Groud from RS2GP, VetAgro Sup
- Séverine Barry from EPIA, INRAE
- Evelyne Picard-Meyer from ANSES
