Agroecology 4 min
First evidence that extracellular nanofilaments manipulate cell shape
PRESS RELEASE - Until now, it was thought that the shape of plant cells was determined only by the hydrostatic pressure within the cells exerted against the cell wall. INRAE researchers, in collaboration with scientists from Cambridge University and Caltech / Howard Hughes Medical Institute, have discovered that the cell wall is an active participant in shaping plant cells. Published in the journal Science on February 27, 2020, these results also have implications in the animal world and could, in the future, inspire the development of new smart self-expanding materials.
Published on 02 March 2020
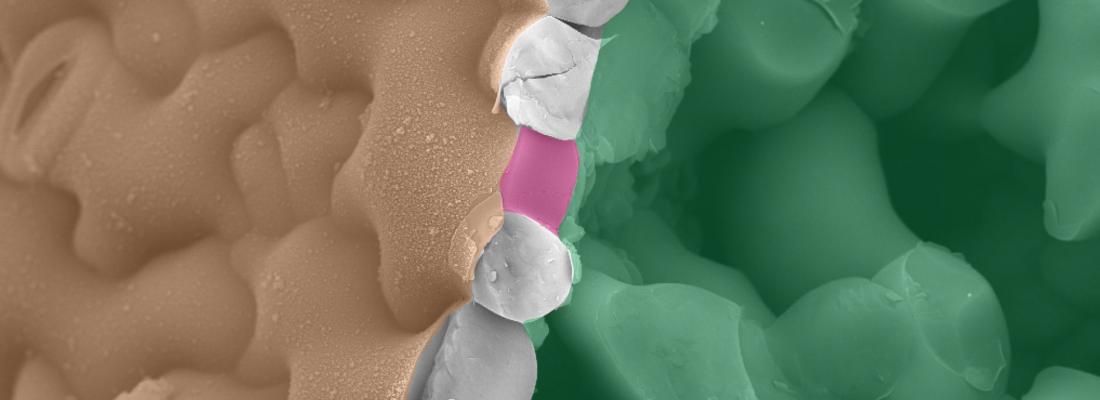
It might look like a uniform surface of green, but place a typical leaf under a microscope and an intricate patchwork of irregular-shaped cells fitting together perfectly like a jigsaw puzzle is revealed. Each of these cells on the surface of a leaf, called pavement cells, has its own unique shape and continues to expand and change shape as the leaf grows.
The current "textbook" thinking about how these unusual wavy-shaped cells are formed is that hydrostatic pressure (turgor) within the cell pushes against the rigid cell wall that surrounds the cell to define its final shape. Weaker parts of the wall expand further, like air pressure forcing weaker areas of a balloon to expand more.
This view has now changed with a team of scientists showing that turgor pressure is not required for the initiation of cell shape – or even cell growth. Researchers from the French National Research Institute for Agriculture, Food and Environment (INRAE) together with scientists from the University of Cambridge and Caltech/Howard Hughes Medical Institute discovered that the cell wall itself is an active participant in shaping plant cells.
The team’s findings, published on 27th Frebuary in the journal Science, include identification of new structures within the cell wall called pectin nanofilaments, which are 1,000 times thinner than a human hair and responsible for driving cell shape independent of turgor pressure. The findings also have implications in the animal world and could inspire future development of smart materials.
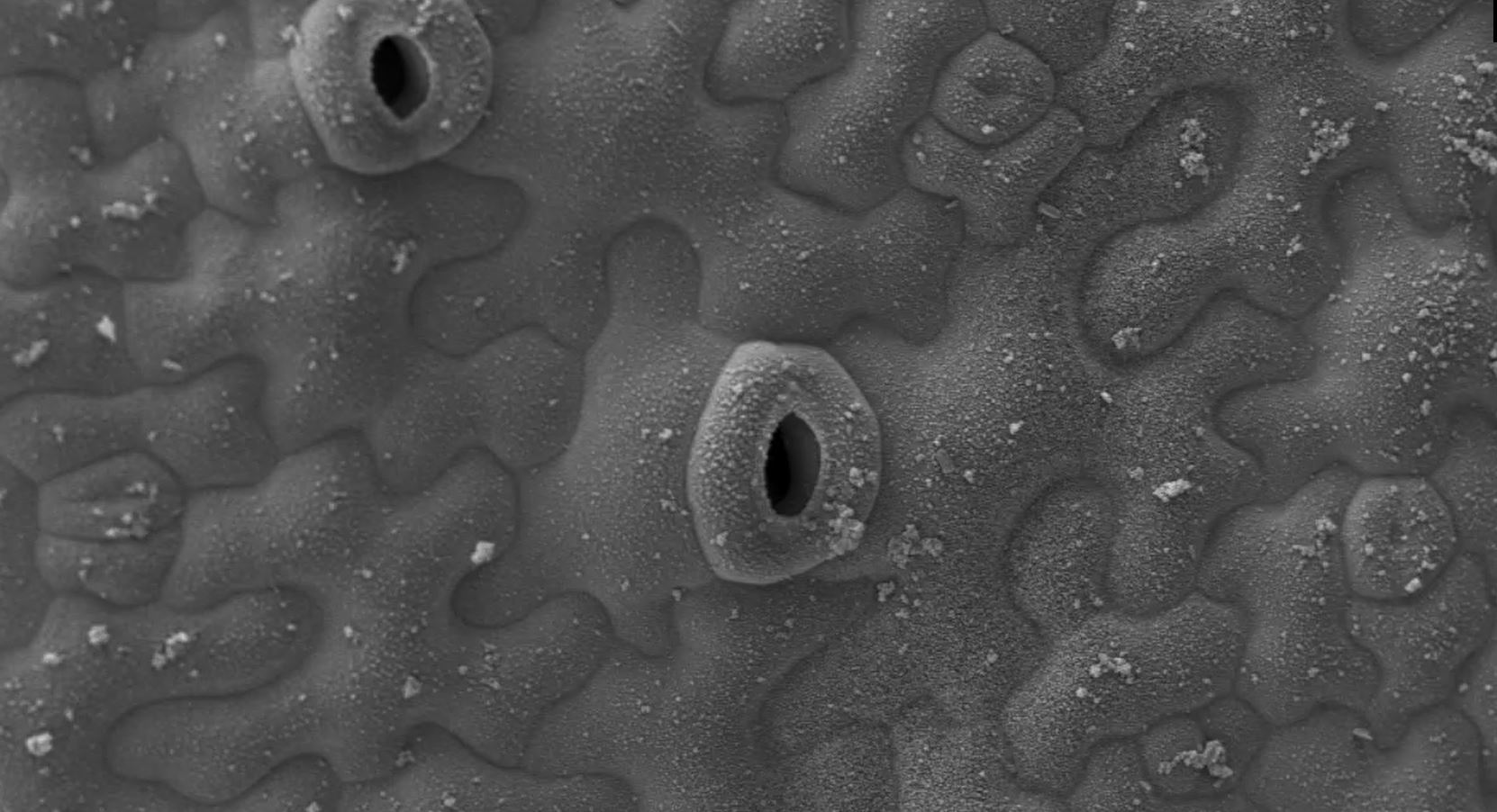
Before the team’s discovery, pectin was considered a disorganised gel-like filling material sitting between the long cellulose fibres in the cell wall. Dr Kalina T. Haas, first author of the paper, now an INRAE researcher, said: “Biochemistry is typically used to study the components of the cell wall, but biochemical analysis disintegrates the cell wall to extract molecules for further study and so we do not get a chance to examine the original structure. Conventional fluorescence microscopes with a resolution of 200 nm aren’t any help either as the cell wall is only 50-100 nm in width and too small for this type of microscope to see its detailed structure. To overcome this, we used two types of cutting-edge microscopy, dSTORM and cryoSEM, which allowed us to keep the cell wall intact. Together, these microscopes revealed that pectins do not form a 'jelly', but create a well organised nanoscaled colonnade (sequence of columns) along the edge of the cell wall.”
The cryo Scanning Electron Microscope (cryoSEM)1 developed at the Sainsbury Laboratory at the University of Cambridge captured the very first images of these pectin filaments. Dr Raymond Wightman, Imaging Core Facility Manager at the Sainsbury Laboratory, said: “It was in a lab 40 years ago that chemists first demonstrated that pectin might form filaments, but these had never been observed in nature. The cryoSEM provided us with the very first images of pectin as filamentous structures and the super-high resolution light microscope called dSTORM confirmed that what we were seeing was actually pectin structures. No single microscope by itself could have confirmed these results.”
Dr Haas and Dr Alexis Peaucelle at INRAE adapted the MRC/LMB’s dSTORM microscope to analyse the leaf cells of Arabidopsis thaliana (thale cress) at a high resolution of 20-40 nm. They found that a single type of chemical change (methyl group removal) in the pectin nanofilament triggers the filaments to swell and expand radially by around 40%. This swelling causes buckling of the cell wall, which then initiates the growth and formation of the unusual wavy-shaped cells.
Dr Peaucelle explains: “This is related to a change in the packaging of pectin polymers inside the nanofilament from a compact to a loose lattice. Such self-expansion of the cell wall components, coupled with their local orientation, can drive the emergence of complex shapes. A computer model found the small change in size that accompanies a modified nanofilament is enough to make the jigsaw puzzle cell shape. Furthermore, these shape changes did not need the force of turgor within the modelled cells.”
Further research will be required to determine what contribution turgor pressure and the cellulose in the cell wall play in determining cell shape. The team think it likely that turgor pressure and cellulose work alongside pectin nanofilaments to help to maintain shape.
Dr Peaucelle continued: “We also found that the puzzle-shape of plant epidermal cells is very ordered. When we sonified the images, we observed that their shapes are organised in waves similar to that produced by a musical instrument. As an example, we used different cells to create notes from a chromatic scale and then play ‘The Blue Danube’ by J. Strauss with them. It is extraordinary that through increasing our understanding of how the epidermal cells form their wavy pattern, we also confirmed that pectin is involved in the growth process. This highlights how little we know about something so vital for sustaining our society as plant growth. I envisage further discoveries in plant and human health will come as more attention is given to the extracellular matrix surrounding cells, thanks to the new generation of high-resolution microscopes. Although animal cells are not surrounded by cell walls, they are surrounded by an extracellular matrix of proteins and sugars, which may similarly guide cell shape.”
Dr Haas concluded: “Knowing the cell wall structure is crucial for understanding how plants form their complex shapes. It is also important in helping to progress knowledge in many areas that will benefit society, such as plant immunity, biofuels, agriculture, plant adaptation to changing environments, and even future development of smart, self-expanding materials.”
1 Scanning at very low temperature
|
Références Kalina T. Haas, Raymond Wightman, Elliot M. Meyerowitz et Alexis Peaucelle (2020) Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells, Science, 27-02-2020. DOI : 10.1126/science.aaz5103 |
|
About the Sainsbury Laboratory at the University of Cambridge The Sainsbury Laboratory Cambridge University (SLCU) is a research institute funded by the Gatsby Charitable Foundation. The aim of the Laboratory is to elucidate the regulatory systems underlying plant growth and development. SLCU has a highly collaborative and interdisciplinary research environment that draws on molecular, cellular, whole plant, computational, quantitative biology/mathematical biology and population biology approaches to investigate the regulatory mechanisms underlying plant growth and development. |

